Before we get started, though, I should say that 2012 wasn't all biology. It started with a physical chemistry lesson--namely, the science of the Olympic Flame. But in the spirit of the Winter Olympics, I thought I'd kickstart my 2014 Science of the Olympics series with a post not about fire...but about ice.
 |
| Sorry, Elsa. I love you dearly, but this is the science--not the magic--of snow and ice. I'll save you for a different blog series. |
THE SCIENCE OF SNOW AND ICE
 |
| Image credit: Michael. |
When water crystallizes in the atmosphere--freezing from tiny suspended liquid droplets into eensy flakes (or frozen fractals, as Elsa would say)--eventually, those flakes fall due to the effects of gravity. And as they fall, any number of things could happen. Below, I've run through the five most common options:
1. The flake could stay cold (below freezing) all the way down. This would create a snowfall.
2. It could warm up on the way down. This could make the crystal flake melt and result in rain.
3. It could warm up on the way down and then get cold again. If it warms to above freezing and cools back to below freezing, this could melt the flake, then refreeze it, creating a new, more ball-like pellet of sleet.
4. It could warm up on the way down, and only get cold again upon reaching the surface of Earth. This would result in freezing rain.
5. It could warm up on the way down, but only a little, and with some added winds this could simply result in snowflakes clumping together to make bigger snowflakes.
 |
| Image Credit: Emmanuel Boutet |
Once snow piles up, its crystal structure reflects light so amazingly well that it will appear white to our eyes. Since the snow doesn't typically break light apart, the white is simply the color of all the wavelengths of the light combined, coming back at us. Occasionally, though, snow looks more blue.
Blue snow can occur when there's a lot of snow around, and light gets scattered bunches and bunches. When this happens, some light absorption does occur, but it's mostly red light that gets absorbed. Therefore, blue is still reflected!
Now, the reason we love snow and ice so much in the Olympics is because they allow for activities that would normally be impossible for humans to achieve.
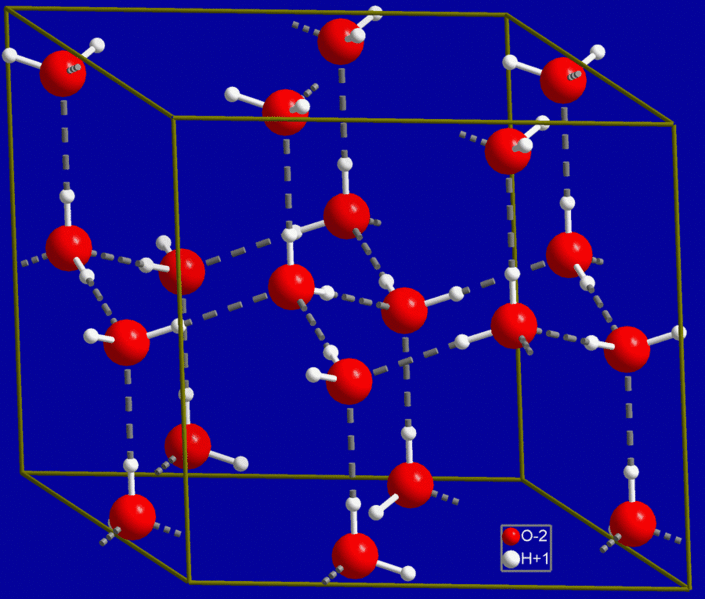 |
| The molecular structure of ice. |
In fact, low friction is pretty much the point of nearly all winter sports. You can't glide over the ground on skis, sleds, or skates without snow and ice! For the time being, though, I'm not going to go into the physics details of all this just yet, because the following blog posts will take a closer look at how exactly these sports work. So stay tuned for that!
In the meantime, I'll leave everyone contemplating this: what if we did the Winter Olympics with one of the other forms of ice? There are 15 known forms, after all, including an amorphous form that has no crystal structure, a highly dense form that would actually sink in liquid water, and a form that can change polarity when exposed to an electric field.
Wacky.
But here on Earth, we typically deal with what is known as ice Ih.That's what allows for ice rinks, ski hills, frost on your glass, and blizzards outside. And that's what will allow for the sheer awesome display of skills over the next few weeks.
So, without further ado... let the Snow and Ice Games begin!

No comments:
Post a Comment